Symptoms of retinal detachment: how to identify them in time
29/09/2025
04/10/2024
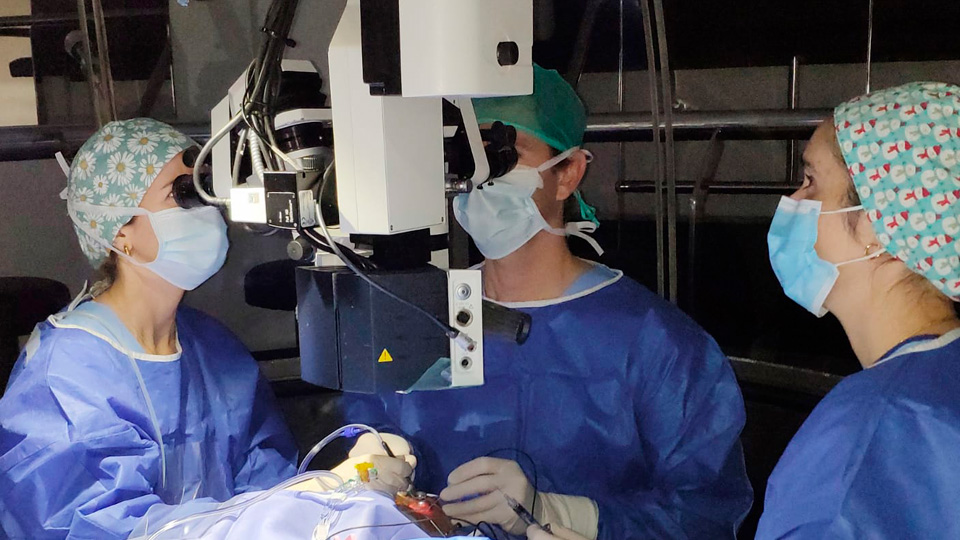
At Barraquer, under the guidance of Dr. Abengoechea, we are conducting an important clinical trial focussed on the treatment of Age-related Macular Degeneration (AMD) in its neovascular or wet form. The “Velodrome” clinical trial, sponsored by Roche Laboratories, aims to evaluate the efficacy, safety, and pharmacokinetics of an innovative slow-release device called the “Port Delivery System®” (PDS), designed for the continuous administration of Ranibizumab a medication already widely used to treat this ocular condition.
The trial, currently in phase 3, is in the process of recruiting patients who meet specific criteria. The ideal candidates are individuals diagnosed with neovascular AMD within the past 9 months, including those already receiving treatment with other medications. This device has generated significant interest due to its potential benefits in terms of comfort and quality of life for patients, who would no longer need to undergo frequent intravitreal injections but could benefit from sustained drug release.
What is the Port Delivery System?
The Port Delivery System® is a device surgically implanted in the sclera. The procedure is minimally invasive and performed under local anesthesia, which significantly reduces recovery time and the risk of complications. Once implanted, the device slowly releases Ranibizumab and in a controlled manner into the eye, allowing for the maintenance of a constant therapeutic dose over a prolonged period.
This system was specifically designed to reduce the frequency of intravitreal injections in the treatment of neovascular AMD. With the PDS®, patients can maintain control of the disease for six to nine months before requiring a new procedure to refill the device. This represents a significant improvement in terms of convenience and adherence to treatment for individuals with this chronic condition.
Ranibizumab, which is administered through the PDS®, is an antiangiogenic drug that works by inhibiting vascular endothelial growth factor (VEGF), responsible for the formation of these new blood vessels. Its controlled administration via the Port Delivery System offers a more efficient and less invasive solution for managing this serious ocular condition.
Patient Recruitment
This clinical trial is open to patients diagnosed with neovascular AMD within the last 9 months, as well as those already receiving treatment with other medications. The duration of the trial and treatment ranges from three and a half to four and a half years, depending on the individual evaluation of each study subject.
During this time, follow-ups will be conducted to assess both the efficacy of the device and the safety and tolerability of the treatment.
This device has already been approved and commercialized by the FDA in the United States. In Europe, the Velodrome trial will provide additional data on its efficacy in different patient groups. Those interested in participating in this study can contact the Barraquer Ophthalmology Centre for more information or visit their website, and get in touch with the Clinical Trials Department.
Dr. Santiago Abengoechea, Principal Investigator of the Velodrome study and ophthalmologist at the Barraquer Ophthalmology Centre
Victòria Hernández Grima, Head of the Clinical Trials Department at the Barraquer Ophthalmology Center."